This article originally appeared on Benzinga.
Clinical trials on the hallucinogenic drug DMT for the treatment of depression are expected to start in January.
According to The Guardian, the UK’s Medicines and Healthcare Products Regulatory Agency (MHRA) gave the green light to the Imperial College of London, in partnership with neuropharmaceutical research company Small Pharma, to begin the trials.
DMT, a strong, short-acting hallucinogenic compound, remains illegal in the UK as in most countries around the globe. It is best known as the active ingredient in ayahuasca, a hallucinogenic drink traditionally used as a spiritual medicine in shamanic ceremonies among indigenous communities in South America.
Studying DMT for Depression
This would be the first time DMT is studied in the treatment of clinical depression.
The institutions stated that the essays will be based on recent clinical research on psilocybin, the active psychedelic compound in magic mushrooms.
In its initial phase, 32 healthy volunteers will be given DMT in order to understand the minimum dose needed to achieve a psychedelic effect. After that, the drug will be administered to 36 separate patients, who have been diagnosed with clinical depression.
The parties still await Home Office approval given the scheduled condition of the drug.
DMT vs Psilocybin: Small Pharma CEO Peter Rands said that DMT’s intense, short-acting psychedelic effect could become a viable alternative to psilocybin treatment in cases where patients cannot participate in the long sessions required by the latter.
A typical psilocybin “trip” will last between two to four hours. In a medical setting, that’s accompanied by four more hours of psychotherapy in order to integrate the experience into the patient’s life.
DMT typically has an onset and offset time of just 15 to 30 minutes, allowing for faster sessions within the long-term treatment.
More Research On The Way
Earlier this year, New York-based MindMed (MEO:MMED) announced it will begin studying DMT’s correct dosing and duration in a Phase 1 clinical trial conducted in collaboration with University Hospital Basel in Switzerland.
More companies are also looking at clinical research on DMT.
On Dec. 9,2020, Entheon Biomedical (CSE:ENBI), a Canadian company focused on studying DMT’s medicinal potential, closed a drug-supply agreement with Psygen Labs Inc.
Entheon will receive DMT for future formulation, preclinical and clinical research, aimed at receiving post-approval commercialization of the drug. The company expects to start clinical trials on DMT by late 2021, in association with the Centre for Human Drug Research in the Netherlands.
Are you still missing out on The Bluntness newsletter? Sign Up today to stay in the loop.







 Complete Guide to Psilocybin Mushroom Strains - The Bluntness Photo by
Complete Guide to Psilocybin Mushroom Strains - The Bluntness Photo by  Complete Guide to Psilocybin Mushroom Strains - The Bluntness Photo by
Complete Guide to Psilocybin Mushroom Strains - The Bluntness Photo by 







 How Long Do Shrooms Last? Magic Mushroom Guide for Beginners - The Bluntness
How Long Do Shrooms Last? Magic Mushroom Guide for Beginners - The Bluntness Psilocybin can provide a life-altering experience. -The Bluntness
null
Psilocybin can provide a life-altering experience. -The Bluntness
null
 “Don’t diddle the dose. Once you have done your homework, go for it.” -- Terence McKenna
The Bluntness
“Don’t diddle the dose. Once you have done your homework, go for it.” -- Terence McKenna
The Bluntness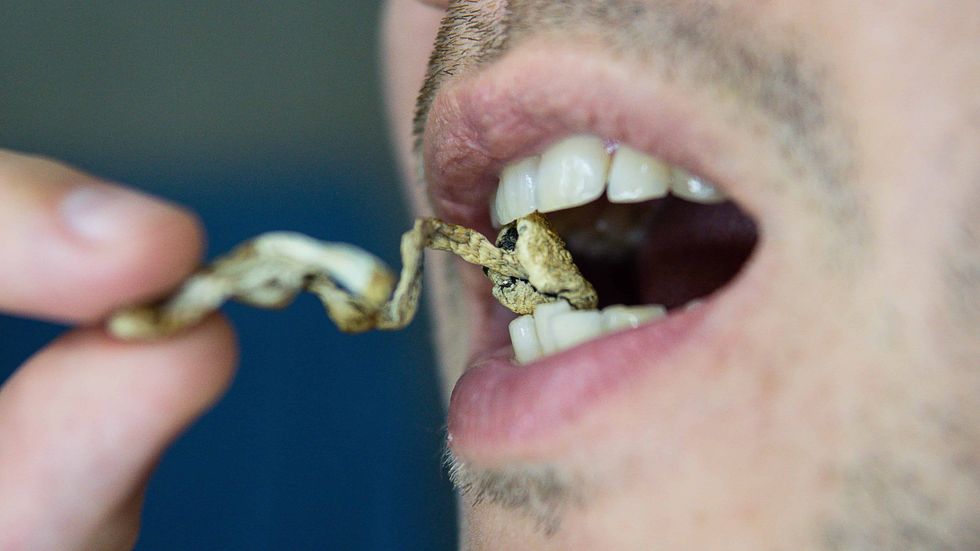 These mushrooms taste gross, but there are ways around that.The Bluntness
These mushrooms taste gross, but there are ways around that.The Bluntness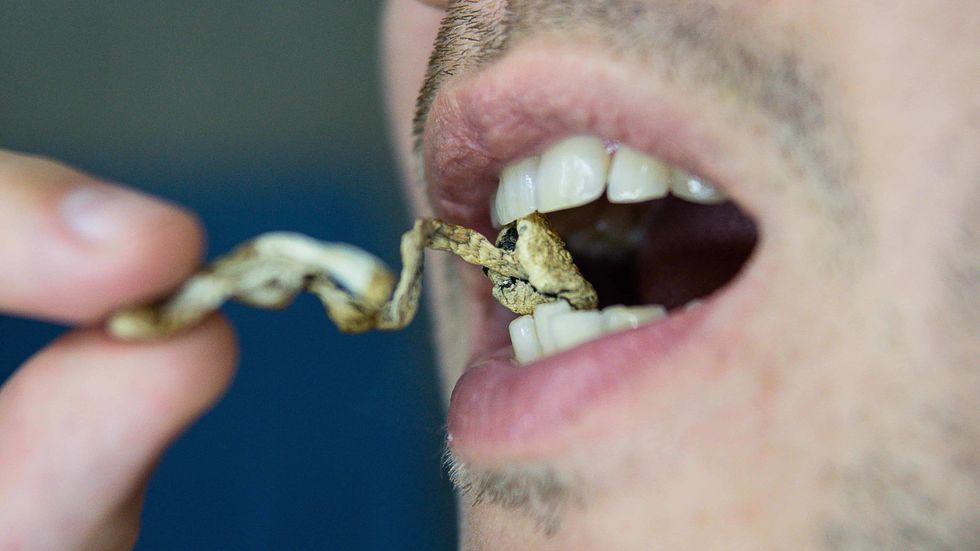 These mushrooms taste gross, but there are ways around that.
These mushrooms taste gross, but there are ways around that.
 How to Make Mushroom Tea - The Bluntness
null
How to Make Mushroom Tea - The Bluntness
null
 How to Make Mushroom Tea - The Bluntness
www.pickpik.com
How to Make Mushroom Tea - The Bluntness
www.pickpik.com
